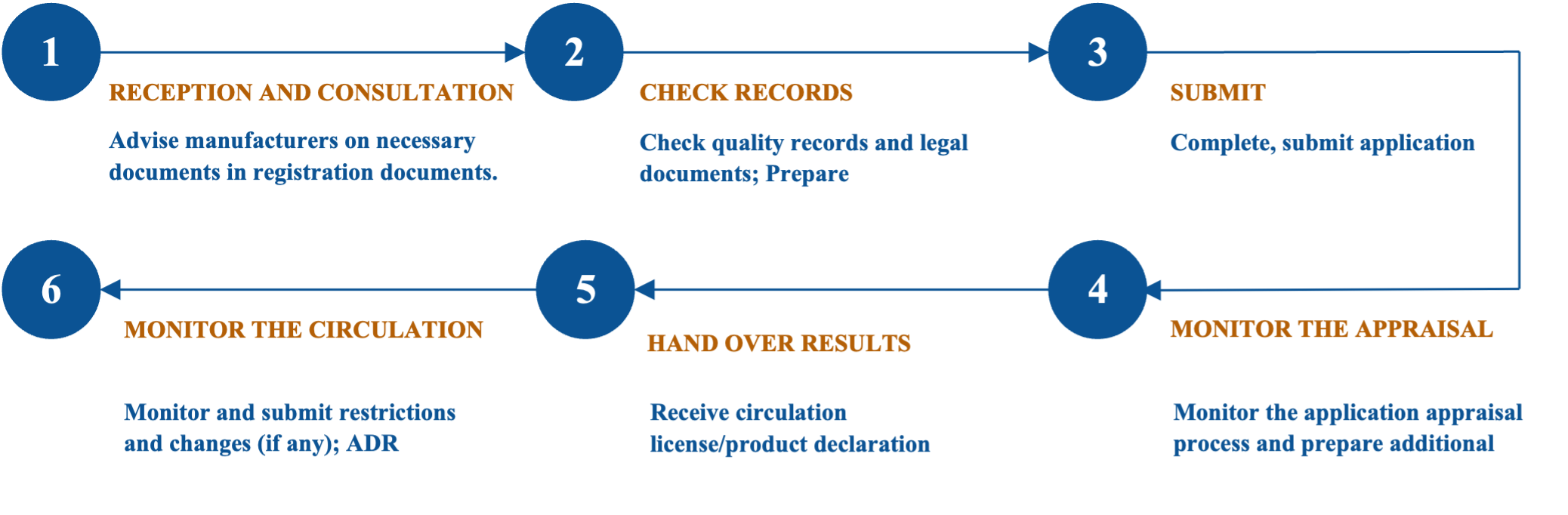
Receiving authority: Department of Health, Medical Equipment and Infrastructure Management Department – Ministry of Health
Categorization:
- Group 1: Medical devices Type A, B – Standard compliance publication
- Group 2: Medical devices Type C, D – Product circulation registration