Consultation process for GPs.
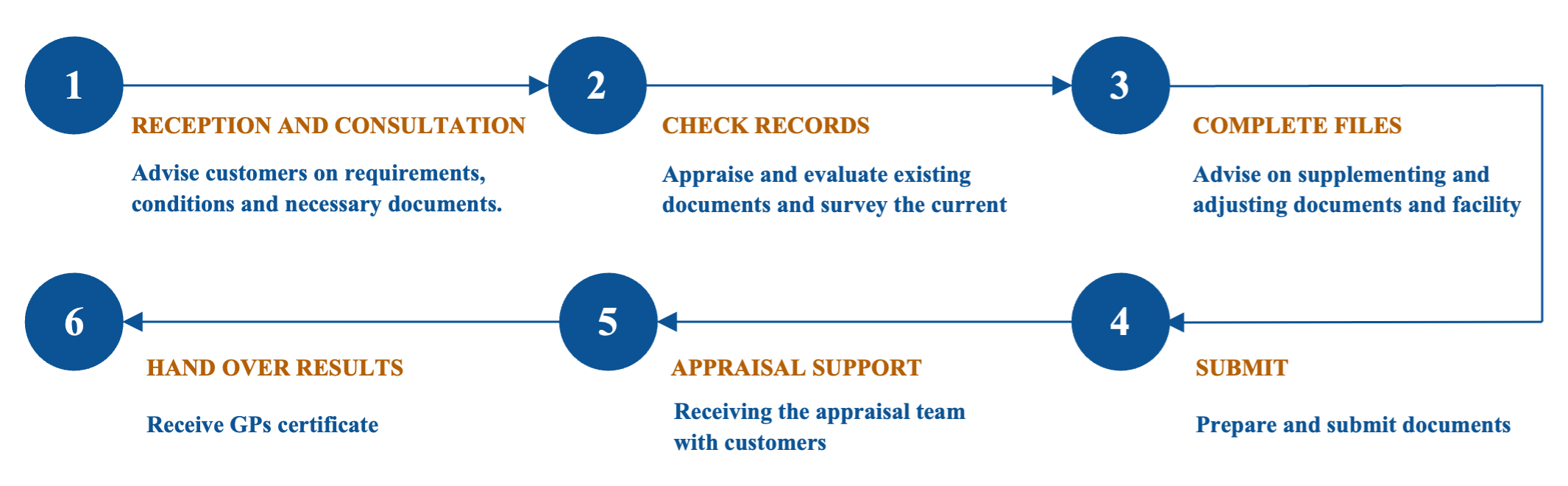
Direct guidance on drafting, completing the necessary documentation as required by the regulatory authority, submitting the dossier, and monitoring the assessment status to obtain the GPs certificate.


Direct guidance on drafting, completing the necessary documentation as required by the regulatory authority, submitting the dossier, and monitoring the assessment status to obtain the GPs certificate.